
.webp)
Acromegaly Support Resources for You and Your Patients

Risa
Watch as Risa describes her journey with MYCAPSSA—and how oral therapy helped bring flexibility back to her adventurous life.

Jim
Listen as Jim talks about life with MYCAPSSA, and the support he's received along the way. Watch as he encourages acromegaly patients to advocate for themselves.

Becky
Watch Becky as she talks about transitioning to treatment with MYCAPSSA, and how she's made it fit into her daily routine.
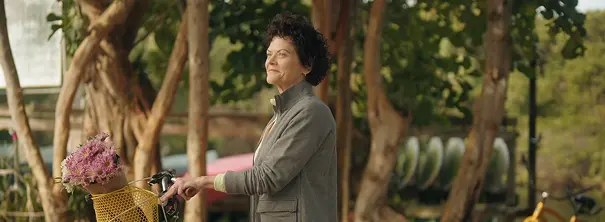
Bonnie
Watch as Bonnie discusses her experience with acromegaly symptoms, and why she decided to change her treatment to MYCAPSSA.

Getting to Know MYCAPSSA
Watch endocrinologist Dr Christofides answer Becky’s questions about MYCAPSSA in this informative Q&A session.
MYCAPSSA is the only oral octreotide.1,2 MYCAPSSA is an oral somatostatin analog approved for the long-term maintenance treatment of acromegaly in people who have responded to and tolerated treatment with octreotide or lanreotide.1
Patients who respond to and tolerate octreotide or lanreotide may be eligible to take MYCAPSSA instead of receiving injections.3MYCAPSSA has an established safety profile consistent with octreotide, without injection-related reactions.4
The most common side effects of MYCAPSSA are headache, joint pain, nausea, weakness, diarrhea, and excessive sweating. GI-related adverse events (AEs) were mostly transient and resolved in less than 2 weeks. Across 2 phase 3 trials, GI-related AEs were reported in 57% and 68% of patients. GI-related AEs were mostly mild to moderate and occurred during the initial 3 months of treatment.1 Learn more about possible side effects in our Safety Profile.
MYCAPSSA is an oral form of octreotide, taken twice a day.1
Somavert® (pegvisomant for injection) is a subcutaneous injection, self-administered once daily after a physician-supervised initial loading dose.6 Somatuline® Depot (lanreotide) is for deep subcutaneous injection only, initially administered every 4 weeks by a healthcare professional.7 Sandostatin® LAR Depot (octreotide acetate) and Signifor® LAR (pasireotide) are intramuscular injections, administered monthly by a healthcare professional.8,9
Patients who respond to and tolerate octreotide or lanreotide may be eligible to take MYCAPSSA.
Yes, MYCAPSSA was proven to effectively maintain normal IGF-I and GH levels in the majority of patients.1,10 Learn more about consistent control and the proven benefits of MYCAPSSA here.
Once MYCAPSSA has been started, evaluate IGF-I, signs, and symptoms, and titrate MYCAPSSA as needed. The starting dose of MYCAPSSA is 40 mg per day (20 mg bid). The majority of patients in clinical trials titrated up to a 60-mg dose or higher.1,4,10,11 Once the maintenance dosage of MYCAPSSA is achieved, monitor IGF-I levels and acromegaly signs and symptoms monthly.1 Encourage patients to keep an open dialogue about how they feel and any potential symptoms they may experience.
*Somavert® is a registered trademark of Pfizer Inc.
†Somatuline Depot is a registered trademark of Ipsen Pharma S.A.S.
‡Sandostatin® is a registered trademark of Novartis Pharmaceuticals Corporation.
§Signifor® is a licensed trademark of Recordati Rare Diseases Inc.
||Please refer to the full Prescribing Information for each product for full Dosage and Administration information.






